Comprehensive Design & Engineering for MedTech
We help companies bring products from idea to market with end-to-end development and expert engineering support reducing costs, avoiding delays, and keeping compliance built in from the start.




We are Impact Technical Resources
Founded in 2012, ITR is a technology design and development company with a local presence in Silicon Valley and a technology hub in Vietnam. We provide Product Development and Technical Expert Augmentation services to help our clients move from early research to market-ready products.
We support our clients at every stage of the product lifecycle from R&D through mass production. Our goal is to help bring devices to market faster, more reliably, and at lower cost.
Designs that balance form, function, and user experience so your product is not only reliable but also intuitive to use.
From PCB design to mechanical housing, we build the core hardware systems that keep your device safe, scalable, and production-ready.
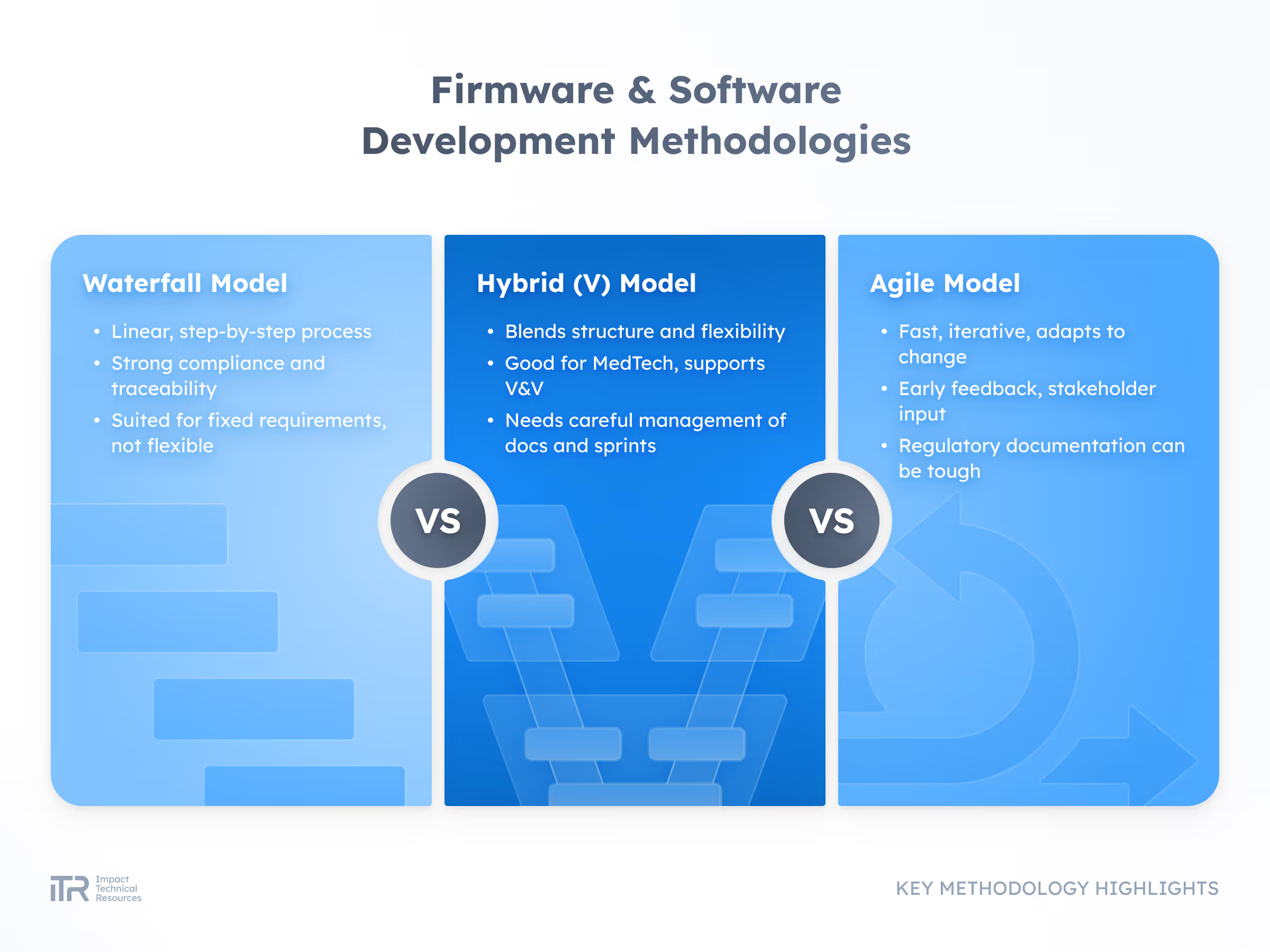
Embedded systems and applications that run smoothly, integrate securely, and meet the performance demands of medical devices.
Cloud platforms and infrastructure built for scale enabling secure data handling, AI-driven insights, and efficient deployments.
Applied research to bring AI/ML to real-world devices, from edge computing to large-scale cloud processing.
Design controls, documentation, and compliance strategies that align with ISO 13485, IEC 62304, FDA, and CE requirements.
Bring Your MedTech Product to Market with Confidence
We take responsibility for the full product journey from early R&D through mass production. Working with a single partner means fewer handoffs, fewer delays, and less risk of miscommunication.
Time-to-Market
We focus on what matters: helping you reach the market sooner without cutting corners. Our development strategies are tailored to your goals, reducing wasted effort and unnecessary costs.
Our team of 100+ engineers combines Silicon Valley experience with deep expertise in compliance and modern technology stacks. You gain the right skills, at the right time, without building a team from scratch.
We understand regulated industries. Our design controls and processes are not just about passing audits they’re about building safer, higher-quality products that last in the market.
MedTech
We design and build medical devices, diagnostics, and monitoring systems with compliance built in from the start.
Our team works under ISO 13485 and IEC 62304 standards to ensure safety and quality at every step. From early R&D to mass production, we help clients bring devices to market faster without compromising reliability.
We also have experience supporting Class II devices through FDA 510(k) clearance, helping clients meet regulatory requirements and prove clinical value.


Digital Health
We create digital health platforms that make care more connected and more personal. Our work spans mobile applications, cloud infrastructure, and AI-driven insights always designed with data security and regulatory compliance in mind.
The result: tools that support remote monitoring, improve patient engagement, and give clinicians the information they need to deliver better outcomes.
Smart Automation
We help companies apply IoT, edge computing, and AI to automate real-world processes. Our focus is on efficiency and measurable results, not just technology for its own sake.
From concept through deployment, we design automation systems that integrate smoothly into existing operations and deliver clear improvements in performance.

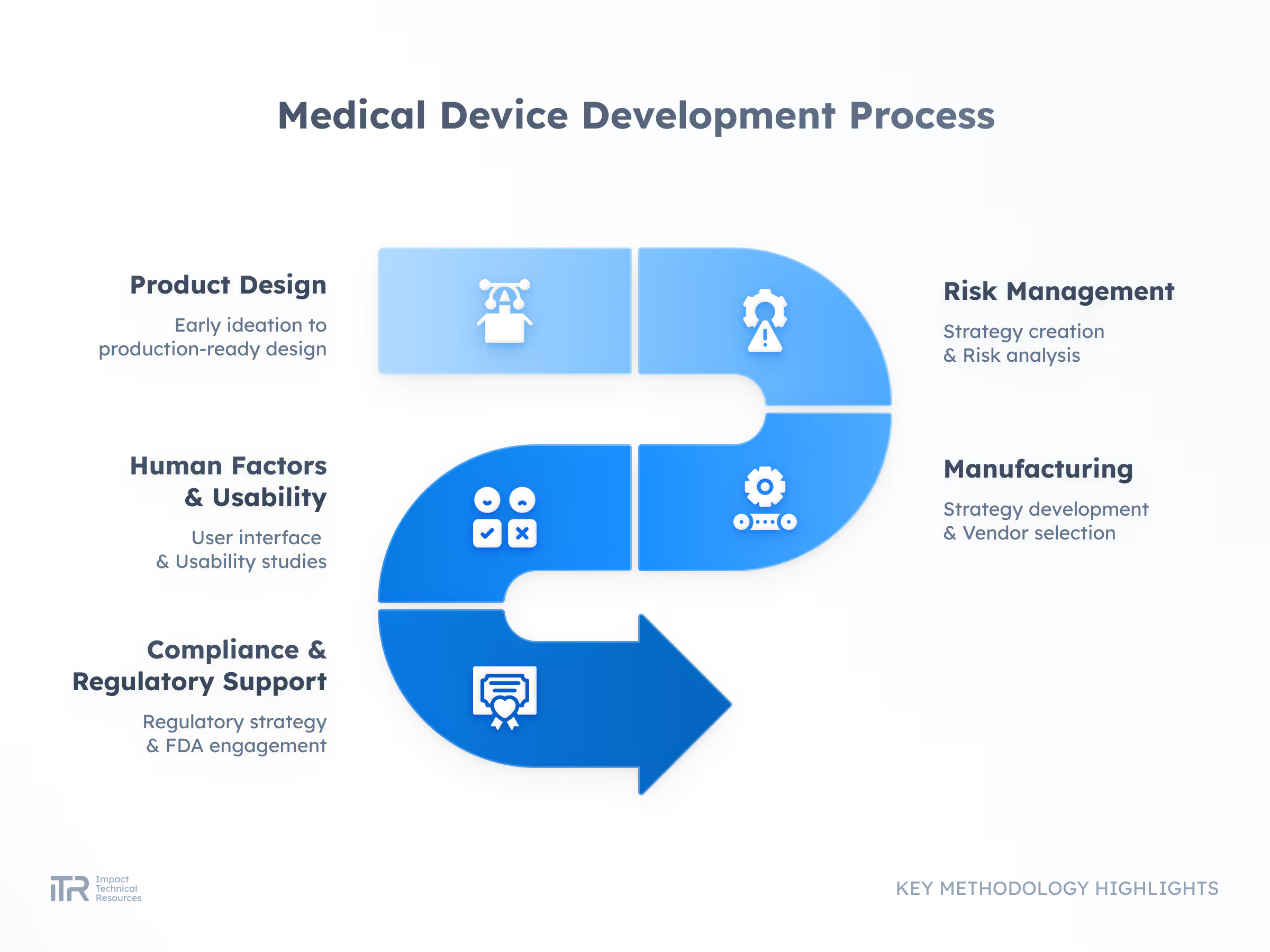
Product Development
From R&D to a market-ready product, we transform concepts into compliant, high-quality solutions. With deep MedTech and wearable expertise, we reduce risks and accelerate delivery.
Product Lifecycle Management
We sustain and evolve your product post-launch—ensuring performance, compliance, and longevity in a changing market.
Project Recovery & Technical Rescue
When projects stall or derail, we step in. Our experts audit, troubleshoot, and re-engineer to bring your product back on track faster and stronger.
Regulatory Support
We prepare robust documentation and guide you through FDA and CE submissions with confidence, aligning your product with the highest regulatory standards.
Industrialization & Manufacturing Readiness
We bridge the gap from prototype to scalable production, optimizing design and supply chains for cost efficiency and smooth manufacturing.
Dedicated Engineering Teams
Scale your R&D capacity with offshore or hybrid models. Our engineers integrate seamlessly with your team, delivering flexibility, speed, and cost efficiency.
Not sure if Our Talents are the Right Fit?
Start with a free 20-hour technical development augmentation to see how our process works. There are no charges or expectations. Fill out this form to get started now.
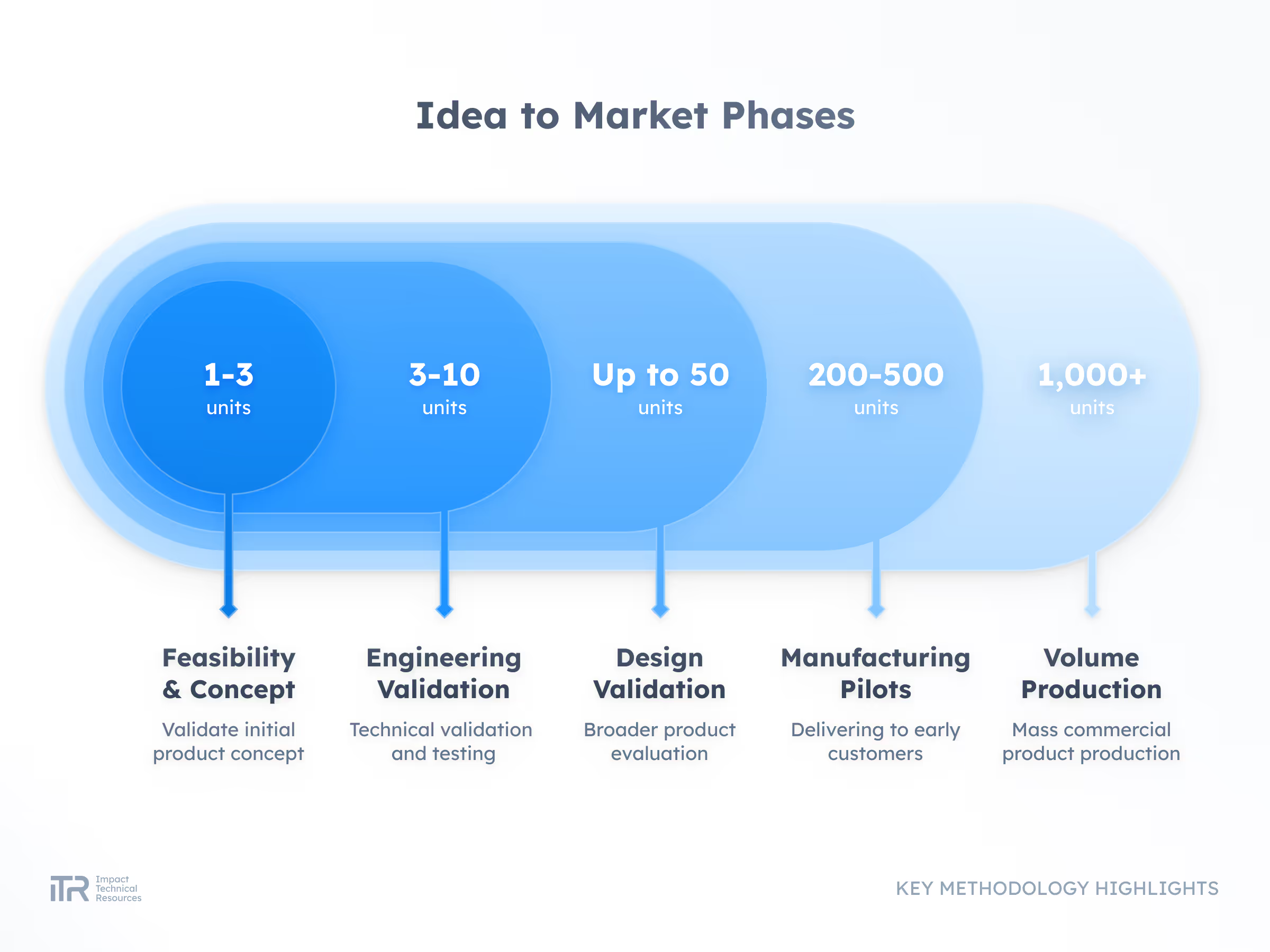
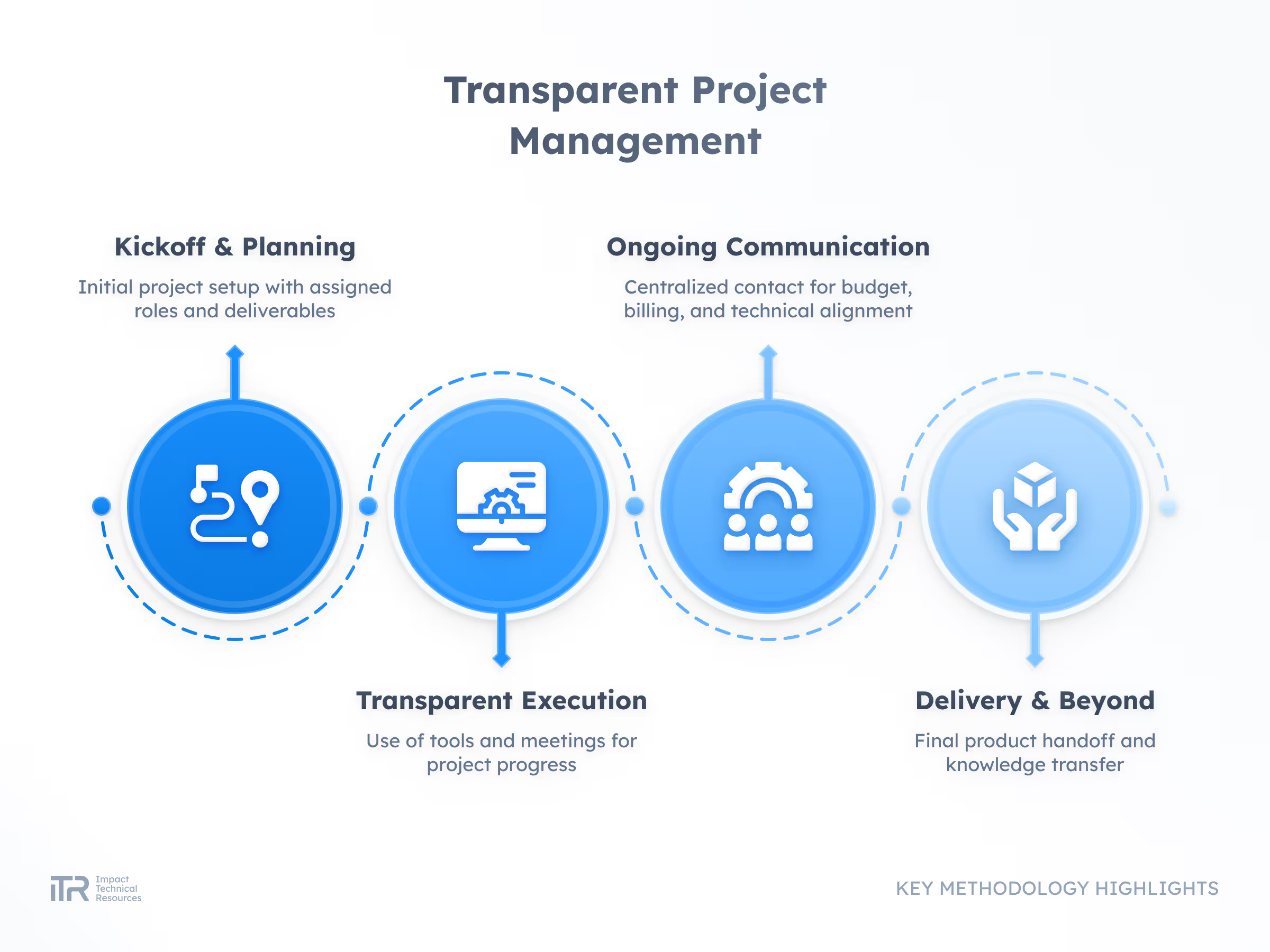
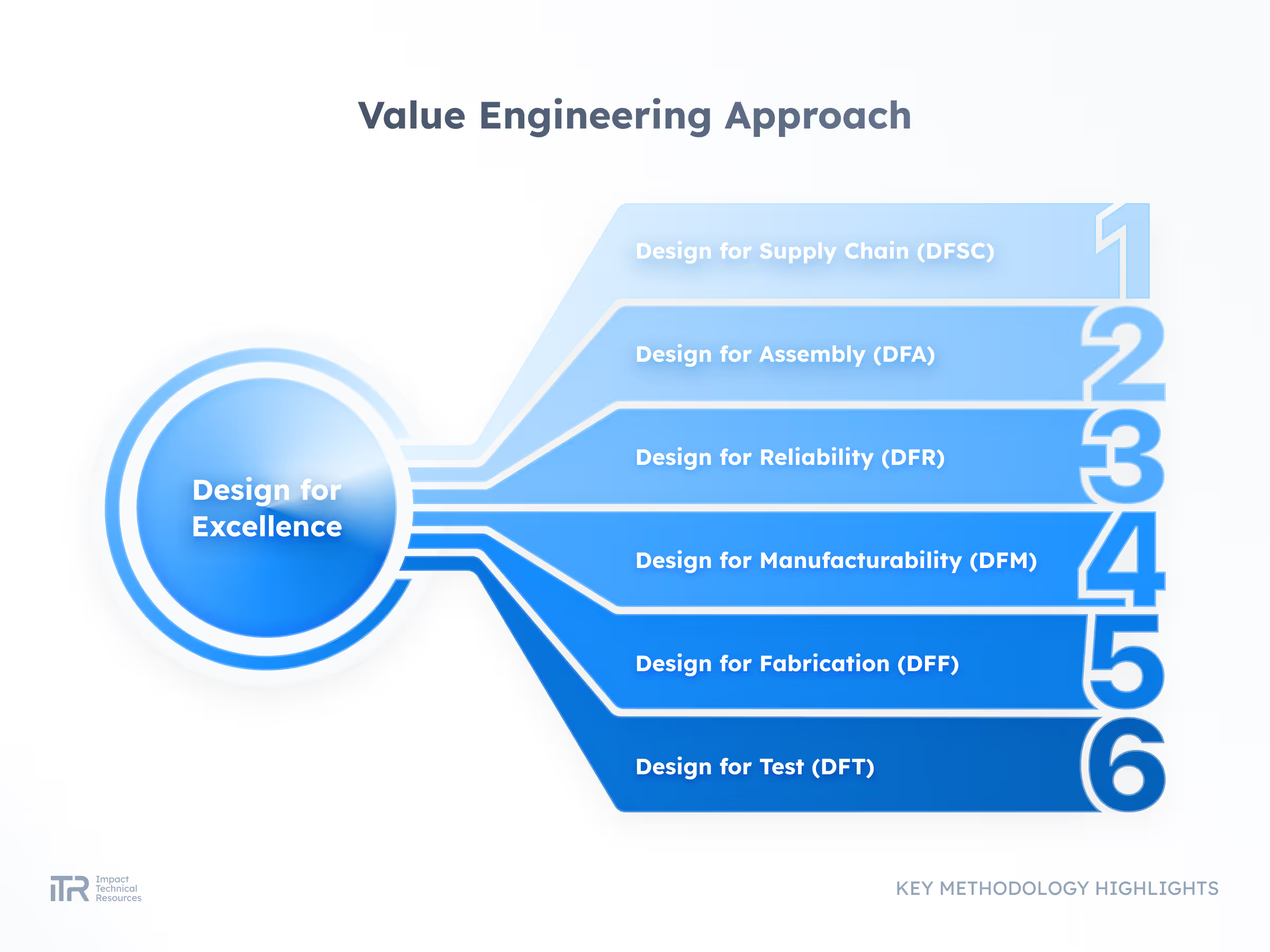
Transforming Ideas into Breakthrough Products
At ITR, we guide your product from concept to mass production with rigor and agility. Our process ensures quality, speed, and compliance with the highest industry standards so your innovation can reach the market with confidence.
Chosen by startups and global MedTech leaders
Clients & Partners

















Compliance & Standards








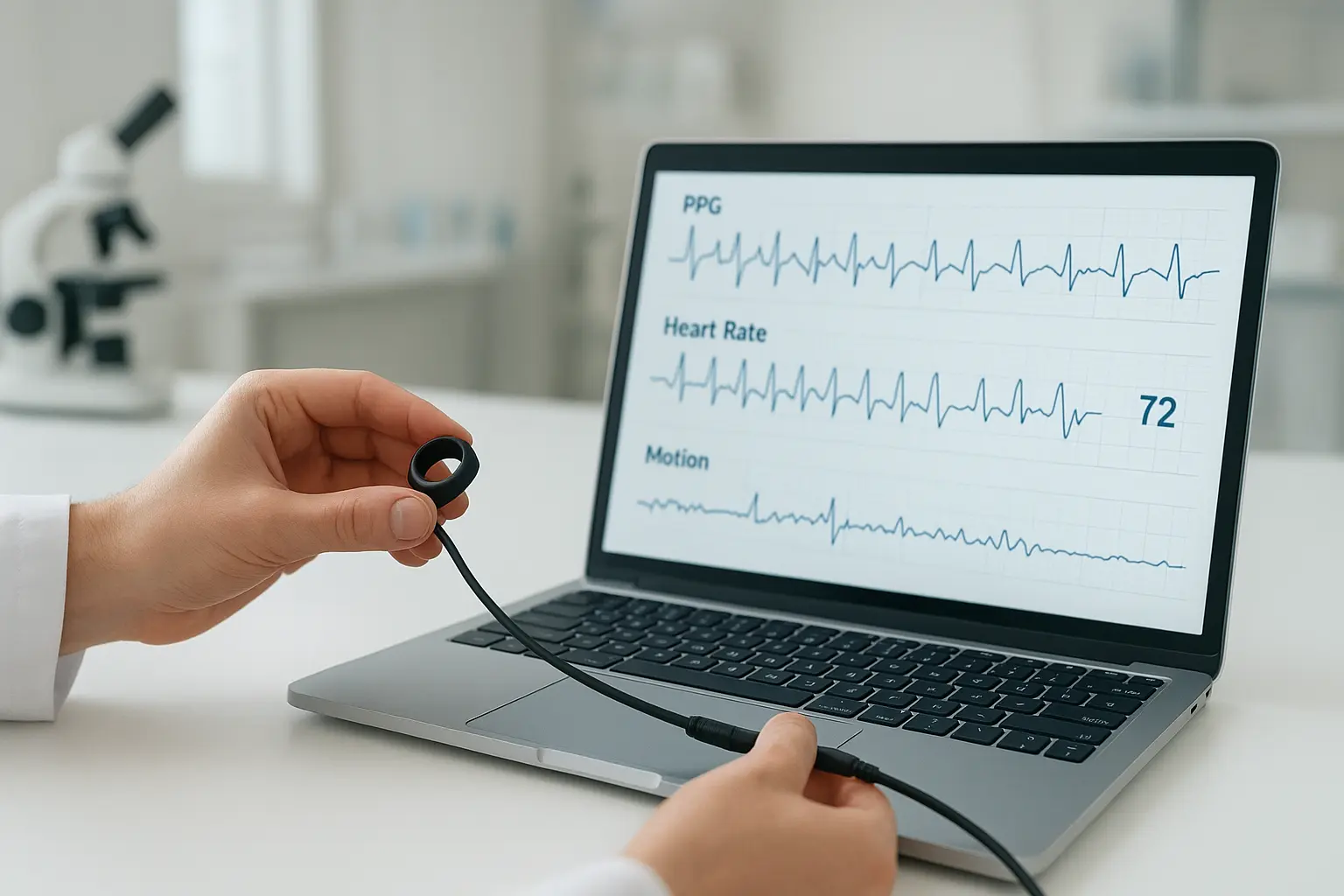
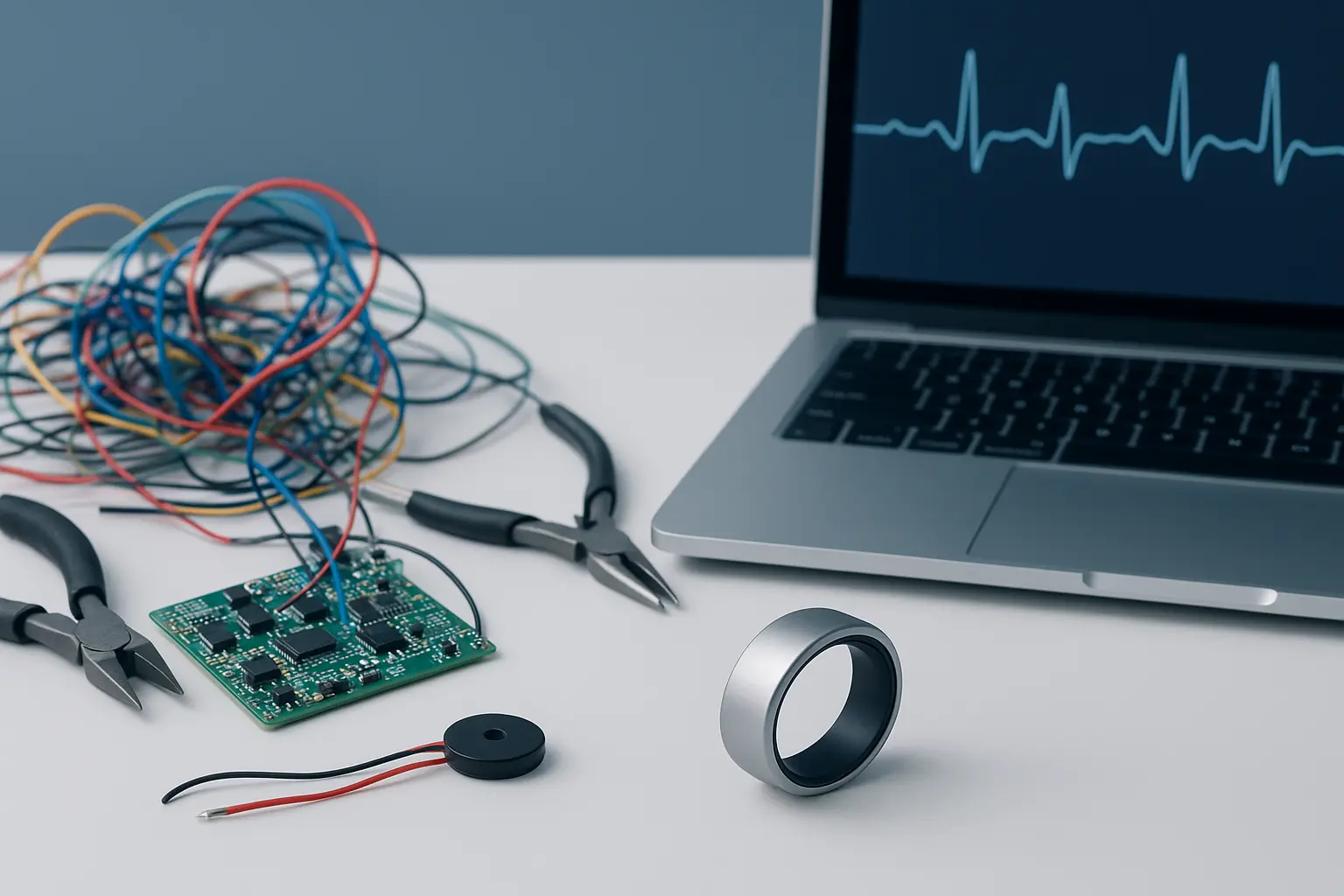
SENSARING - A Biosensor Ring Platform for MedTech Innovators – Powered by ITR’s Engineering Services
ITR’s Biosensor Ring is a medical-grade wearable designed for continuous health monitoring with high-precision sensors, energy-efficient hardware, BLE connectivity, NFC charging, and on-device AI. Unlike consumer smart rings, it meets strict regulatory standards (ISO 13485, IEC 62304, IEC 60601-1), ensuring accuracy and compliance for healthcare applications like sleep studies and fitness tracking. By providing a ready-to-use platform, ITR accelerates MedTech innovation, helping companies bring certified products to market faster.

Smart Patient Remote Monitoring System
ITR's SPRM (Smart Patient Remote Monitoring) System is an IoT platform for remote monitoring of vital signs and cognitive data in COVID-19 patients. It offers multiple vital signs monitoring, data analysis, robust security, seamless integration, multi-connectivity options, and Contact Tracing. This system improves patient outcomes, enables timely alerts, and enhances healthcare accessibility, addressing critical pandemic challenges.

FDA-Cleared ACM System
ITR has developed an FDA-Cleared Ambulatory Cardiac Monitoring (ACM) system, offering a comprehensive solution for diagnosing heart disease. The system consists of a wearable ECG monitoring device with advanced connectivity options, cloud infrastructure powered by AWS, and proprietary AI algorithms. It provides real-time data analysis, secure storage, and convenient access through mobile apps and a web portal. The ACM system enhances early diagnosis, reduces healthcare costs, and improves patient outcomes, revolutionizing cardiac monitoring. ITR's solution meets all regulatory standards and holds significant potential in the US healthcare market.

IoT-enabled Water Coolers from Q&C
Q&C Water Coolers in Hungary offers an innovative IoT-enabled water cooler system that lets users monitor and control their water consumption via a mobile app. Their solution incorporates efficient heating and cooling technologies and self-diagnostics. It's managed through a centralized Q&C Cloud Platform and Portal and allows over-the-air firmware upgrades. The company has secured funding from various sources and received innovation awards. ITR provides services for hardware device design, embedded system development, user mobile app development, and manufacturing quality control mobile app development.
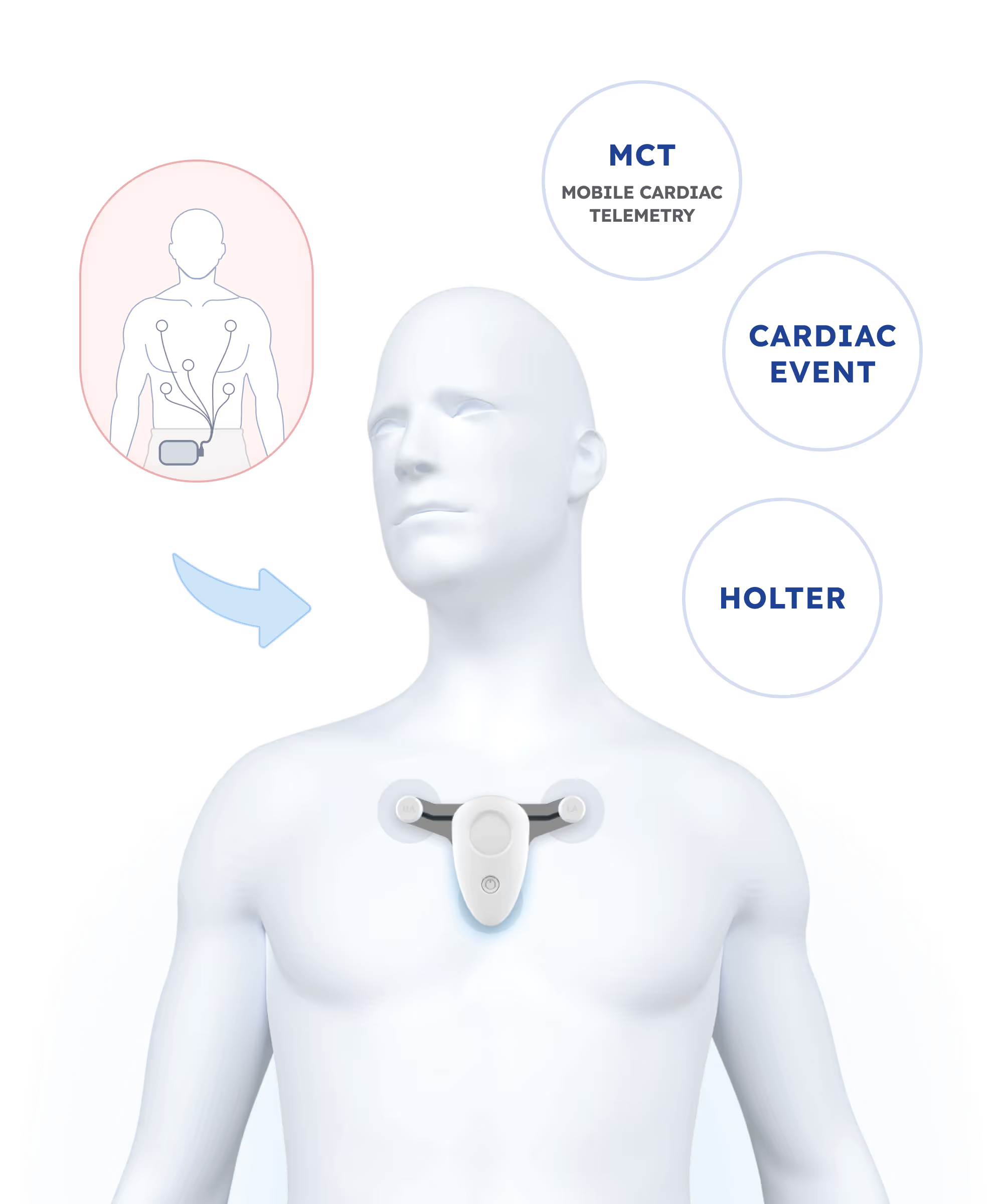
ECG Solution Development
Accelerate your path from concept to FDA-cleared ECG device with ITR’s expertise in Edge AI, wearable biosensors, and regulatory compliance.
All case studies
Powered with years of experience in Digital Health and MedTech, ITR knows how to help our clients push through processes to deliver their products to the world at high levels of quality.