Services
Comprehensive Engineering Solutions to Realize Your Vision
From the first sketch to full-scale production, ITR provides end-to-end engineering that balances speed, compliance, and quality. Our teams ensure every stage is aligned with your business goals, so your product not only reaches the market faster but also thrives once it’s there.
.avif)


Our Core Engineering Disciplines
Full-Cycle Product Development
From idea to launch, ITR manages every stage design, prototyping, testing, and validation to accelerate your path to market while ensuring compliance and quality at each step.
Product Lifecycle Management
We maintain and evolve your product post-launch, ensuring it stays reliable, compliant, and competitive as markets and technologies advance.
Regulatory Supports
We help you navigate global compliance and regulatory submissions, simplifying approvals while ensuring your product meets the highest safety and quality standards.
Industrialization & Manufacturing Readiness
We bridge the gap between design and production, optimizing for manufacturability and scalability to reduce costs and minimize delays.
Project Recovery & Technical Rescue
We step in when projects stall or go off track auditing, redesigning, and executing a recovery plan to restore momentum and deliver results.
Dedicated Engineering Teams
Scale your R&D capacity quickly with our skilled engineers. We integrate seamlessly with your workflows, giving you flexibility and speed while you retain full control.
Full-Cycle Product Development
Driving ideas from concept to market
We help founders and product leaders move from concept to clinical-grade reality.
Our approach combines system-level design, hardware/firmware/software co-development, and regulatory integration from day one. Each phase feasibility, prototyping, V&V, and scale-up is structured with traceable deliverables, reducing risks and avoiding rework.
Our approach combines system-level design, hardware/firmware/software co-development, and regulatory integration from day one. Each phase feasibility, prototyping, V&V, and scale-up is structured with traceable deliverables, reducing risks and avoiding rework.
Capabilities include:

Product Lifecycle Management
Ensuring performance and longevity post-launch
A device’s value is proven post-launch. We provide sustaining engineering and lifecycle support to keep products safe, compliant, and competitive.
We manage:

Regulatory Supports
Bridging compliance gaps with expertise
Compliance isn’t an afterthought. We integrate regulatory requirements directly into the development flow.
Our team builds and maintains Design History Files, Risk Management Files, and Verification/Validation evidence aligned with ISO 13485, ISO 14971, IEC 62304, MDR, and FDA QSR.
Our team builds and maintains Design History Files, Risk Management Files, and Verification/Validation evidence aligned with ISO 13485, ISO 14971, IEC 62304, MDR, and FDA QSR.
Services include:

Industrialization & Manufacturing Readiness
Optimizing design for efficient production
Scaling from prototype to production requires more than design it demands manufacturability. We work with your CM (or help you select one) to ensure smooth handoff and predictable ramp-up.
Focus areas:

Project Recovery & Technical Rescue
Rescuing projects and restoring momentum
Not every project runs as planned. When milestones slip or costs spiral, we step in.
Our process begins with a technical audit covering design files, firmware code, and regulatory status then delivers a practical recovery plan.
Our process begins with a technical audit covering design files, firmware code, and regulatory status then delivers a practical recovery plan.
Typical interventions:

Dedicated Engineering Teams
Expanding capacity with skilled professionals
We extend your R&D capacity with engineers who work as part of your roadmap, not as detached contractors.
Each team is built around your needs HW, FW, SW, AI/ML, QA and is integrated with your sprints, PM workflows, and compliance processes.
Each team is built around your needs HW, FW, SW, AI/ML, QA and is integrated with your sprints, PM workflows, and compliance processes.
Engagement models:

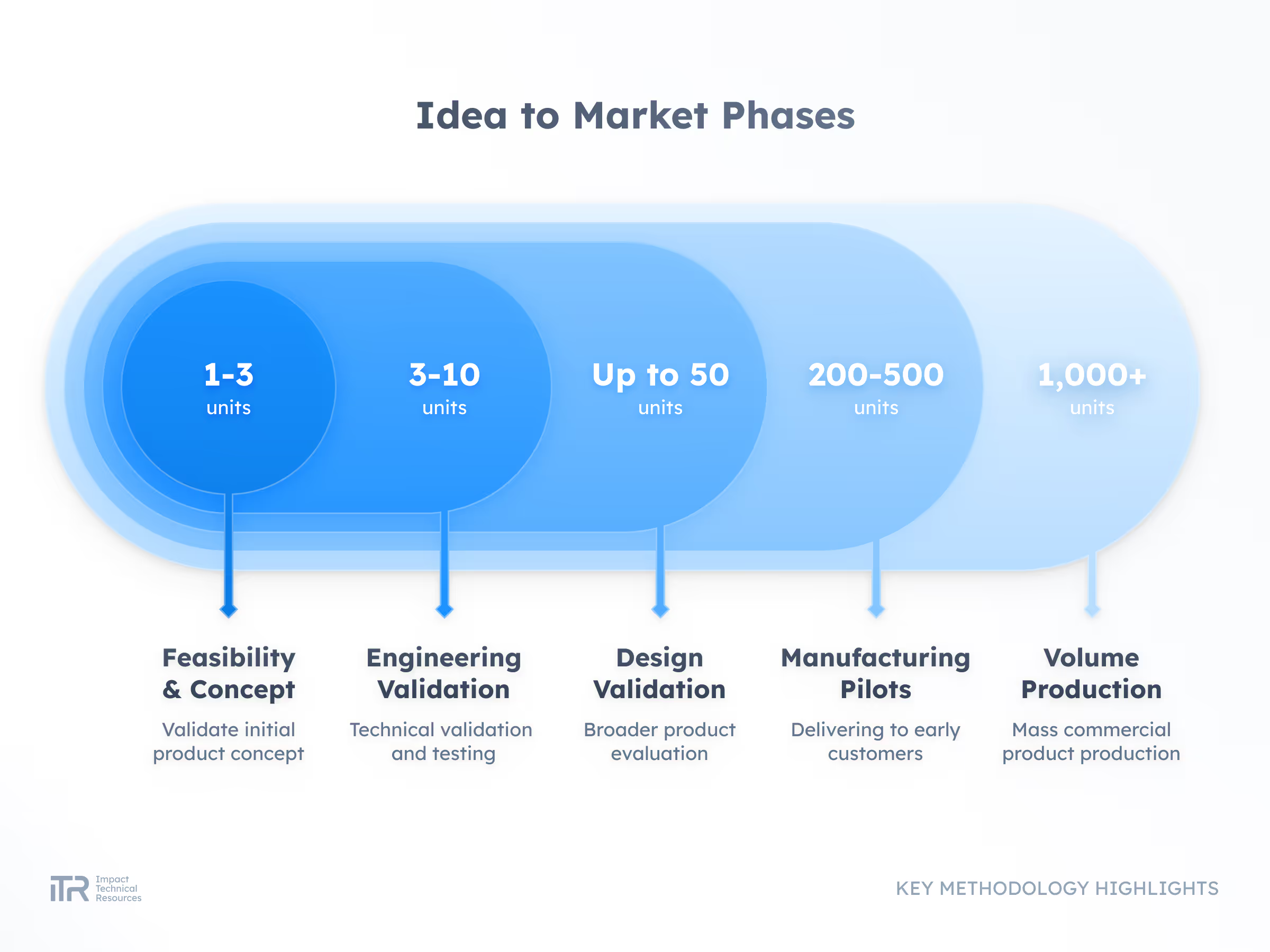
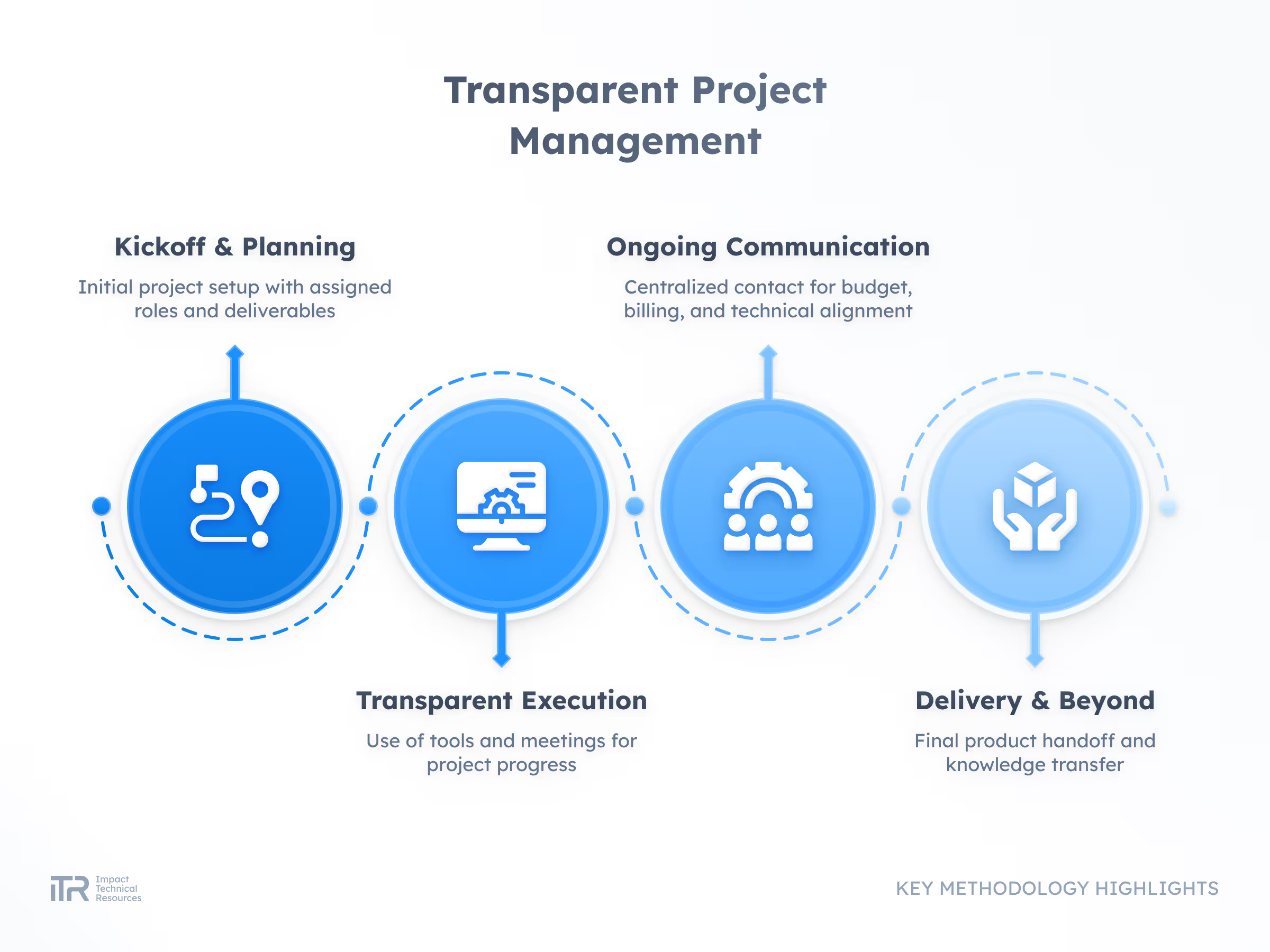
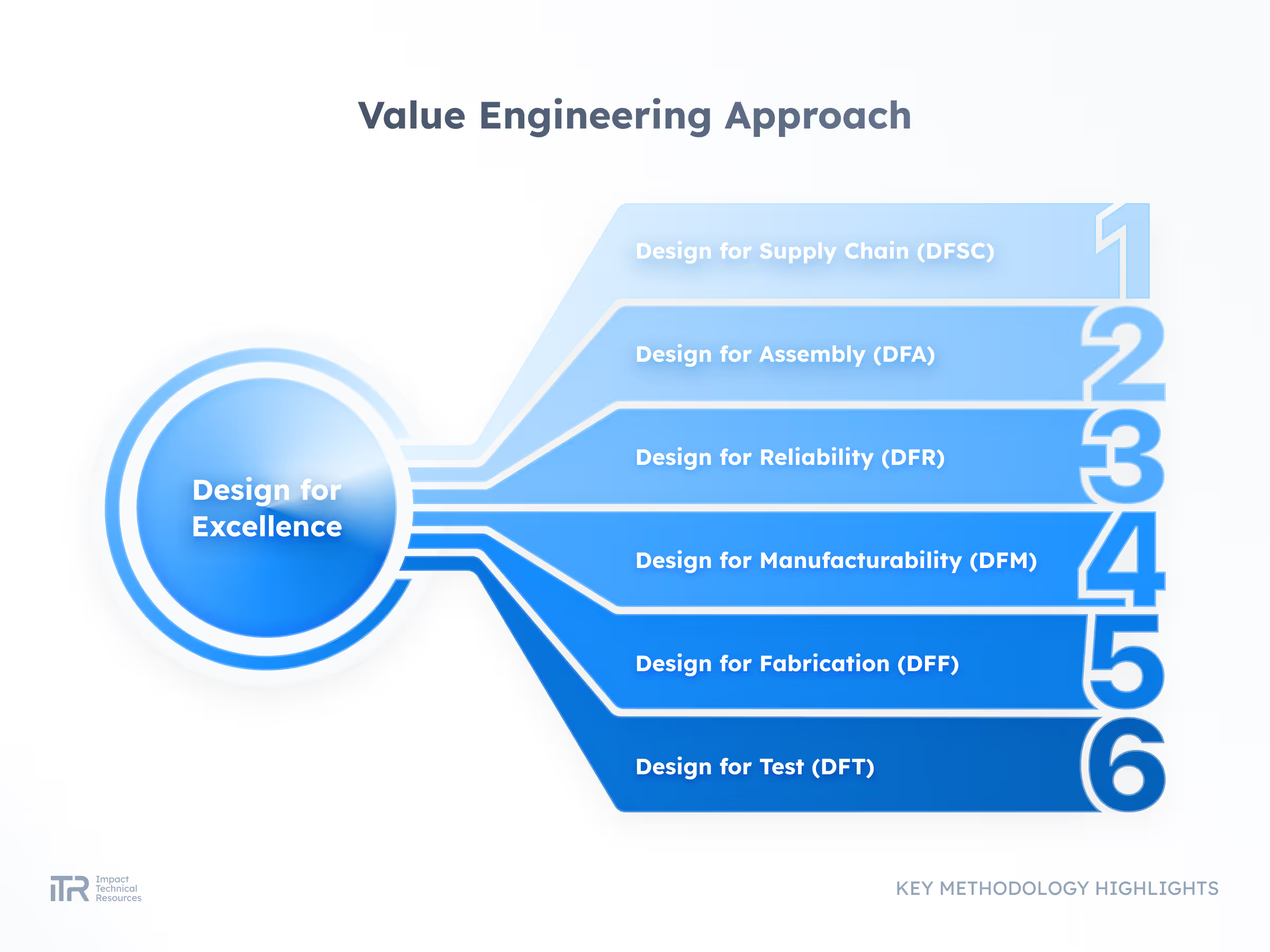
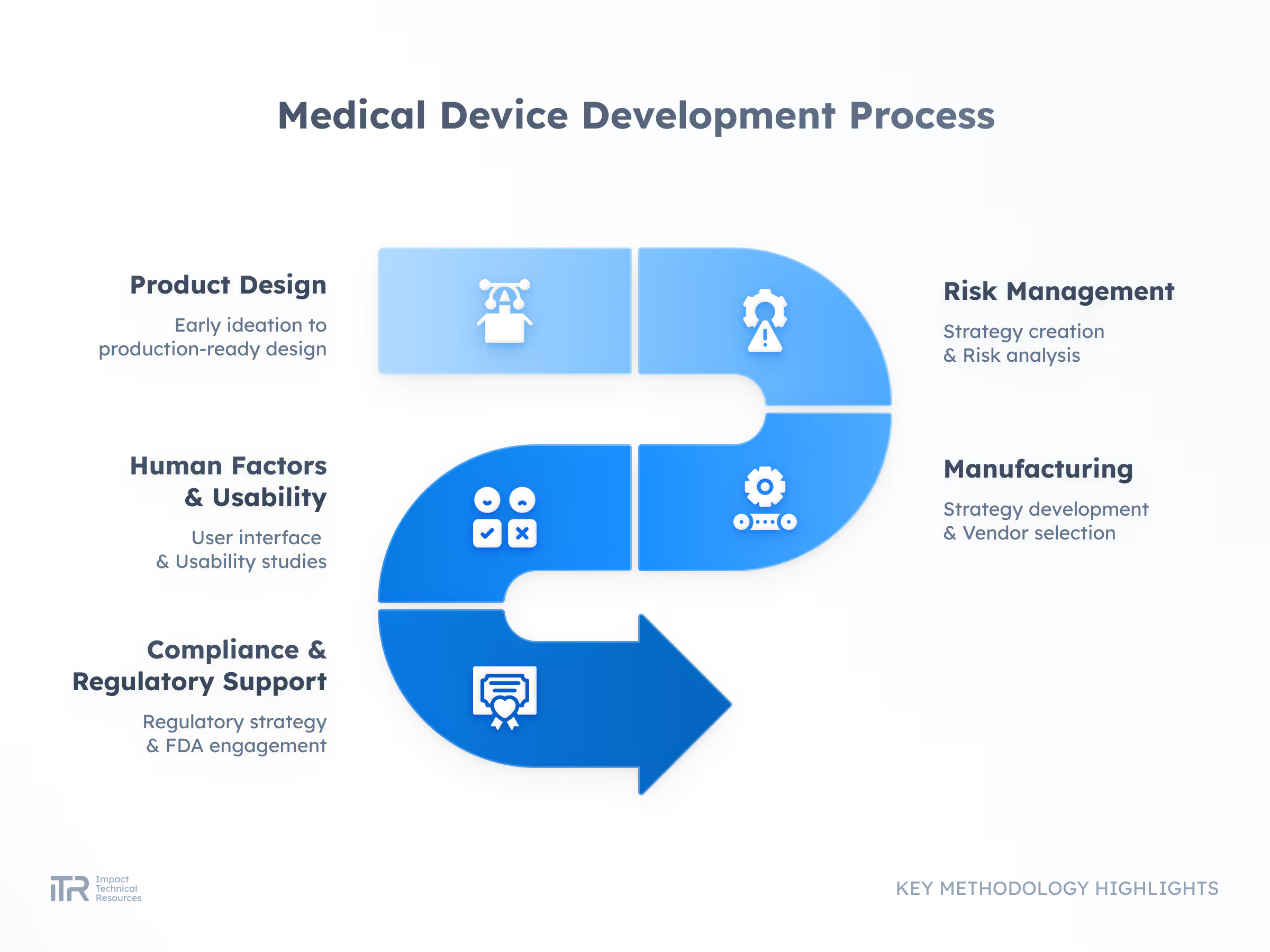
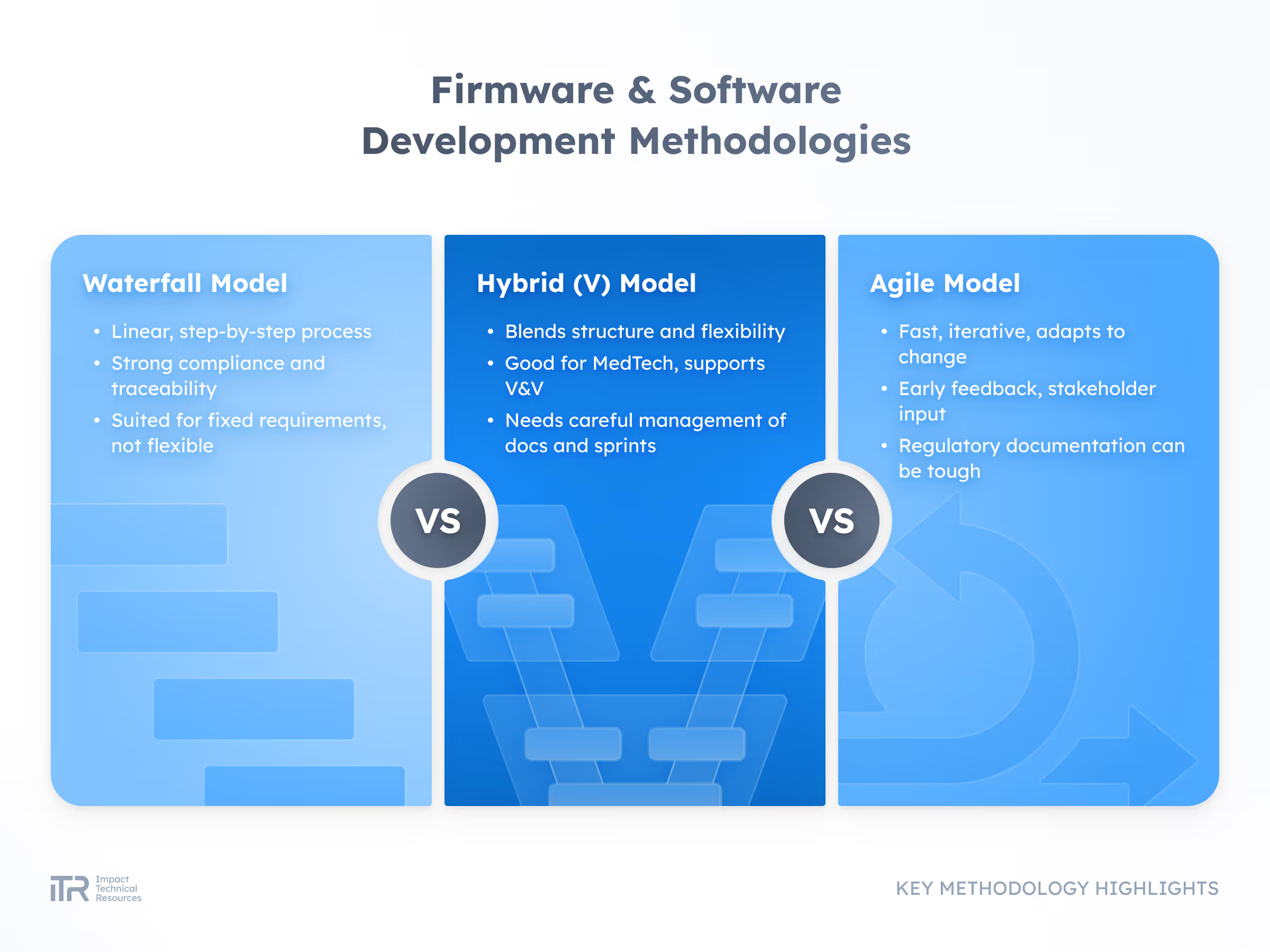
ADVANCED METHODOLOGIES
Transforming Ideas into Breakthrough Products
At ITR, we guide your product from concept to mass production with rigor and agility. Our process ensures quality, speed, and compliance with the highest industry standards so your innovation can reach the market with confidence.
Trust & Proof
Chosen by startups and global MedTech leaders
Clients & Partners

















Compliance & Standards







