

In the highly regulated world of medical devices, balancing innovation, quality, and cost efficiency is a critical challenge. A Design for Cost (DfC) approach ensures that a product meets performance, safety, and compliance standards while remaining cost-effective from development to mass production. This strategy involves optimizing design, materials, processes, and supply chain decisions to minimize costs without sacrificing quality.
Why Design for Cost Matters in Medical Devices

Medical devices must comply with strict regulations (ISO 13485, IEC 60601, FDA 510(k), MDR), making cost reduction a complex task. Unlike consumer electronics, medical device design must prioritize safety, reliability, and long-term stability—often increasing costs. However, an effective DfC strategy allows manufacturers to manage these constraints while ensuring affordability, market competitiveness, and profitability.
Key Cost Drivers in Medical Device Development
- Regulatory Compliance Costs
- Extensive documentation, clinical trials, and FDA/MDR approvals increase costs.
- Failure to plan for regulatory requirements early leads to expensive redesigns.
- Component and Material Selection
- High-quality, biocompatible materials (ISO 10993) drive up costs.
- Custom-designed PCBs, biosensors, and ultra-low-power chips add to expenses.
- Manufacturing Complexity
- Multi-layer PCB assemblies, miniaturized wearables, and intricate enclosures raise production costs.
- Limited availability of specialized manufacturing processes increases pricing volatility.
- Supply Chain and Logistics
- Global shortages of semiconductors and medical-grade materials affect costs.
- Custom parts with long lead times disrupt production timelines.
- Testing and Quality Assurance
- EMC/EMI testing, accelerated life testing, and biocompatibility studies require significant investment.
- High-reliability requirements (e.g., implantables) further increase costs.
Strategies for Cost Optimization in Medical Device Design
1. Early-Stage Cost Modeling

Starting with a Total Cost of Ownership (TCO) analysis helps anticipate expenses across design, manufacturing, and post-market phases. This includes:
- Bill of Materials (BOM) cost projections
- Tooling and production setup expenses
- Compliance and certification costs
Example: A wearable ECG patch manufacturer conducted an early cost analysis and identified a 10% cost reduction by selecting an alternative ISO 10993-certified adhesive instead of an expensive biocompatible polymer.
2. Smart Component Selection

- Use off-the-shelf components instead of costly custom designs whenever possible.
- Prioritize multi-sourced components to avoid supplier dependency.
- Evaluate low-power microcontrollers (MCUs) and optimized wireless modules to reduce power consumption and extend battery life, minimizing operational costs.
Example: By switching from a custom RF chip to a pre-certified Bluetooth Low Energy (BLE) module, a MedTech startup saved $500,000 in NRE costs and accelerated time to market.
3. Efficient PCB and Mechanical Design

- Minimize PCB layer count to reduce fabrication costs.
- Simplify mechanical enclosures by designing snap-fit assembly instead of using screws or adhesives.
- Optimize trace routing and component placement to avoid excessive testing and debugging costs.
Example: A handheld medical scanner reduced PCB costs by 25% by consolidating multiple sensor boards into a single, high-density PCB design.
4. Design for Manufacturability (DFM) & Assembly (DFA)
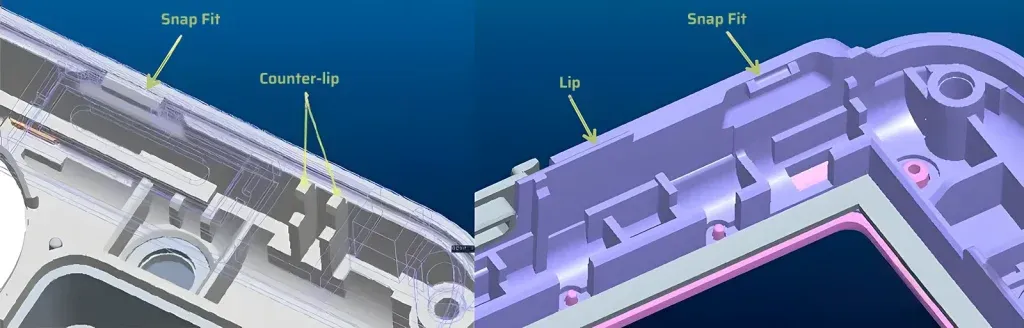
- Reduce manual assembly by designing for automated production.
- Minimize tight tolerances that increase precision manufacturing costs.
- Consider modular designs to allow easy upgrades and reduce obsolescence risks.
Example: An implantable neurostimulator optimized its connector design to allow robotic soldering instead of manual assembly, cutting labor costs by 30%.
5. Regulatory-Driven Cost Planning

- Engage regulatory consultants early to avoid late-stage design changes.
- Design products to align with FDA Class II instead of Class III if clinical use allows, significantly lowering approval costs.
- Use pre-approved components that already meet ISO 13485 and IEC 60601-1 standards.
Example: A startup reduced its 510(k) submission timeline by 6 months by choosing an FDA-cleared battery supplier, saving both compliance costs and time.
6. Supply Chain and Lifecycle Cost Optimization
- Select readily available materials and components to avoid procurement delays.
- Engage with Contract Manufacturers (CMs) early to ensure cost-effective supply chain integration.
- Design for scalability, ensuring low production costs for large volumes.
Example: A telemedicine device manufacturer switched from custom injection molding to a standardized plastic housing, reducing upfront tooling costs by $100,000.
Balancing Cost with Quality and Innovation
While cost reduction is crucial, compromising on quality, safety, or usability can result in regulatory failures, recalls, and reputational damage. A well-structured Design for Cost strategy ensures that medical devices remain affordable, scalable, and compliant, while still delivering high-performance solutions.
At ITR VN, we help MedTech companies implement cost-effective, high-quality product development strategies by leveraging:
- Early-stage cost modeling
- DfM and DFA expertise
- Regulatory and compliance planning
- Strategic component and supplier selection
By integrating Design for Cost principles from concept to mass production, we ensure that our clients develop market-ready, competitive medical devices with optimized manufacturing costs.
Looking for expert guidance on cost-effective MedTech development? Contact ITR VN today to explore how we can help you design smarter, build faster, and scale efficiently.




%20(1).png)